As we travel from one place to another we rarely give much thought to the water we drink, cook with, and bath in. But there are occasions when something seems a little “off” with the water. Sometimes it’s the taste, a hint of hydrogen sulfide, which makes for a very bad cup of coffee, or perhaps the water just seems to be too metallic in taste. More often though a problem with the water reveals itself in one of two ways:
• I can’t generate any soap suds in the shower or
• The opposite happens and I can’t seem to rinse the plentiful soap suds off leaving my skin feeling a little slippery, soapy, and unclean.
What we can never keep straight are the simple reasons for each of these two scenarios. So we are going to write about it here. Hard water, found in 85%, is generally preferred for drinking due to its better taste, makes it difficult to generate soap suds because the soap interacts with the calcium, magnesium, and other minerals in the water. This interaction prevents the soap from performing an effective clean when it comes to dirty clothes or dishes. Hard water leaves a residue behind creating a soap film in baths and showers and scale in piping and water heaters.
Soft water on the other hand, having replaced the calcium and magnesium ions with salt (or potassium), which might make the water taste a little salty, leaves your skin feeling slippery as you rinse the soap off. Surprisingly, that slippery feeling is an indication your skin is clean! Soft water doesn’t have mineral interference, resulting in the need for less soap in addition to achieving cleaner clothes, dishes, and skin.
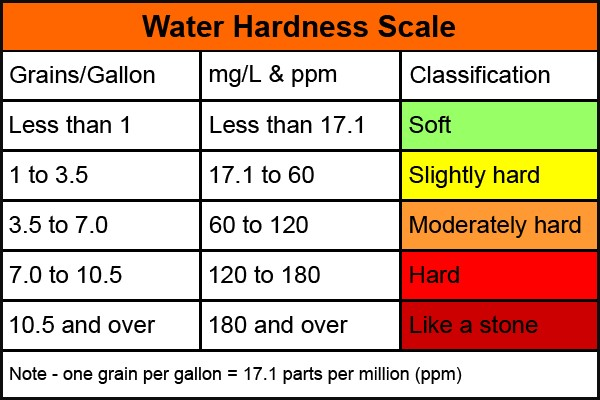
Disadvantage of Hardness
• Hard water does not pose a health threat
• Hard water can contribute to dry skin and hair.
• Hard water is unfit for washing as it is difficult to form lather with soap
• Trimming of kettles will take place due to the formation of magnesium and carbonates of calcium.
• Blocks hot water pipes, which is caused by the appearance of layers of carbonates of calcium and magnesium
Types of Hardness
• Permanent hardness :- Permanent hardness refers to the mineral content in water that is not possible to remove through boiling. The hardness is typically caused by the presence in water of magnesium sulfates and/or calcium sulfate that do not undergo precipitation at increased temperatures. Therefore, permanent hardness is the sum of magnesium hardness and calcium hardness.
• Temporary hardness :- Temporary hardness is water hardness due to the presence of calcium and magnesium carbonates and bicarbonates, which can be precipitated by heating the water. It can be removed by processes such as boiling or lime softening, and then separation of water from the resulting precipitate. Temporary hardness is very common and is responsible for the deposition of calcium carbonate scale in pipes and equipment. These deposit formations lead to clogged plumbing and reduced efficiency of heat exchangers. Temporary hardness is also known as carbonate hardness.
Effect of Hardness
• Skin and Hair
• Appliances and Plumbing
• Soaps and Detergents
• Clothing and Fabrics
• dishes and glass